AIMS (ADVANCED IMMUNOMODULATING MULTI-COMPONENT SYSTEM)
Protective immunity and resolution to health (homeostasis) is orchestrated in parallel by multiple cell subsets, surface receptors, soluble factors and intracellular pathways. One cell, molecule or linear pathway is rarely the sole agent of immune dysfunction (disease). Years of research with naltrexone and met-enkephalin confirmed their anti-cancer and anti-inflammatory properties are a result of immune modulation through multiple cell types, receptors, and pathways. Leveraging that experience, STATERA developed its core development platform, AIMS.
- to rapidly assess how novel compositions impact multiple pharmacokinetic-pharmacodynamic relationships, cellular/molecular selectivity, and overall therapeutic potency;
- to discover with potentially greater accuracy, through methodical property analysis, targets with the improved activity and levels of therapeutic benefit not achieved by other published immunotherapies that use a single target approach;
- to formulate a new class of immune-restorative drugs that harmonize the immune orchestra (by targeting complex pathways and engaging toll-like receptors);
- to comprehensively engage the immune system such that our compositions treat disease, and help resolve inflammation, restoring balance, rather than a one-dimension approach of blunting or exaggerating a single factor.
STAT-200 AIMS Program
STAT-200 analogs are undergoing or have performed in numerous phased trials in autoimmune and infectious disease. AIMS continues to expand the number of novel STAT-200 compositions/uses.
| Discovery | Lead Optimization | Pre-Clinical | Phase 1 | Phase 2 | Phase 3 | NDA |
STAT-200: noroxymorphone-n-substituted-methyl cyclopropyl analogs
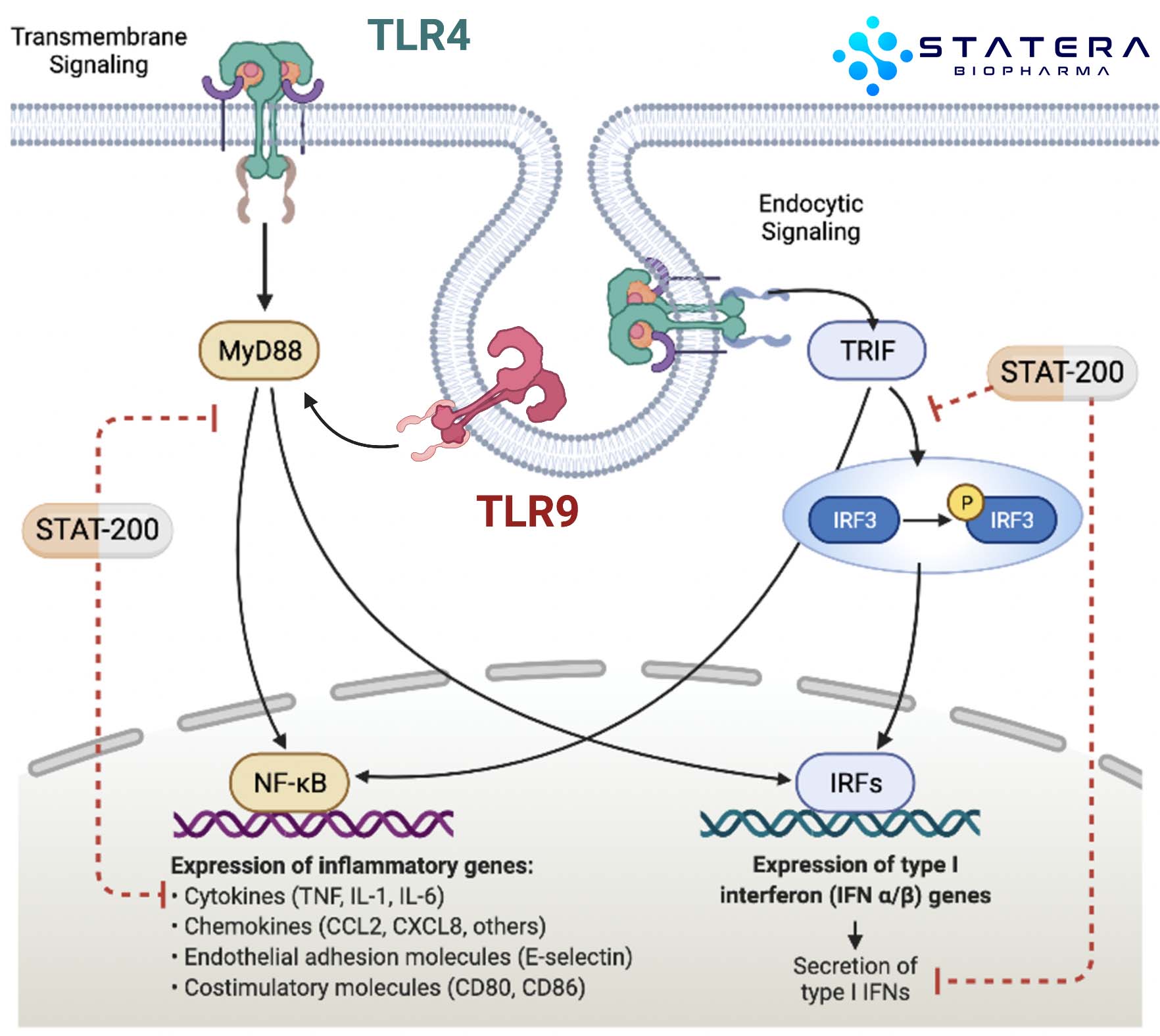
Original discoveries demonstrated anti-angiogenic and anti-inflammatory properties of STAT-200. Clinical observations preceded the complete understanding of the modes of action that drove beneficial immune modulation. Through the AIMS platform, we are geometrically expanding our understanding of these mechanism and developing purpose-built analogs which improve clinical benefit and expand indications of use, thus more fully leveraging their all-important pleiotropic effects. Though the biology is complex, AIMS allows us to integrate multiple factors that define a superior composition including key pharmacokinetic-pharmacodynamic relationships, potency, and selectivity. Through methodical property analyses that utilize informatics, in vitro/in vivo modeling and patient-derived clinical samples, we are defining new targets and compositions with improved properties that modulate the immune response and potentially deliver clinical benefit at levels not achieved by other published immunotherapies.
PLEIOTROPIC EFFECTS: Years of research have revealed parallel mechanisms of action that synergize. STAT-200 compositions modulate the OGF-OGFr axis (homeostatic regulator of cell proliferation), as well as Toll-like receptors (TLRs) and intracellular signaling pathways which regulate inflammatory cytokines and multiple cellular compartments.
STAT-600 TLR5 Program
| Discovery | Lead Optimization | Pre-Clinical | Pivotal Animal Studies | Human Saefty / Dose Conversion |
| Discovery | Lead Optimization | Pre-Clinical | Phase 1 | Phase 2 | Phase 3 | NDA |
STAT-600: Toll-like Receptor 5 agonists
BEST IN CLASS TLR-5 AGONIST
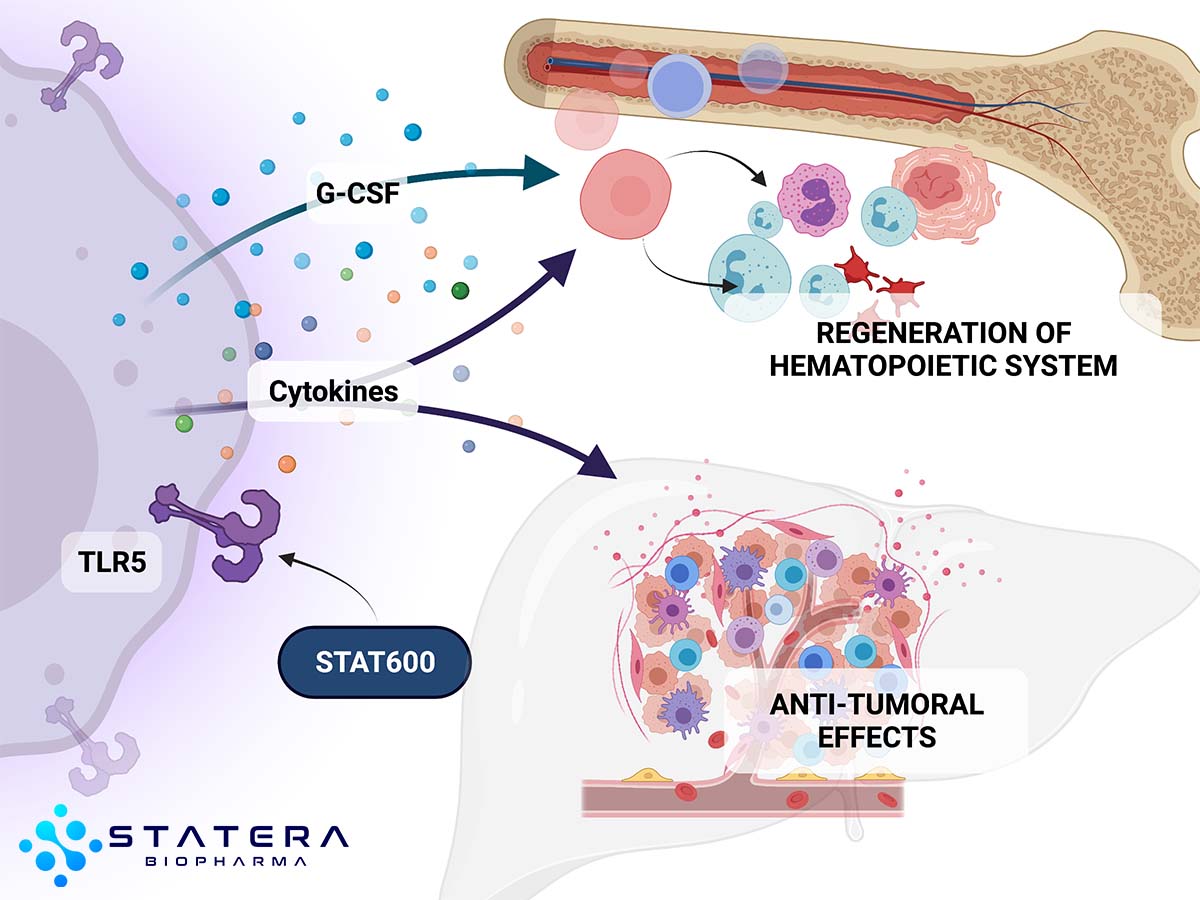
Originally developed for emergency use, the STAT-600 therapies are analogs targeting a much broader set of indications including: hematology, acute radiation syndrome, cancer, and infectious diseases. STAT-600 therapies are a recombinant protein agonist of toll-like receptor 5 (TLR5), an innate immunity receptor. STAT-600 therapies activation of TLR5 triggers NF-kB signaling, mobilizing an innate immune response that drives expression of numerous genes, including inhibitors of apoptosis, scavengers of reactive oxygen species, and a spectrum of protective or regenerative cytokines.
STAT-600 Oncology and Hematology: The STAT-600 program looks to leverage the data obtained from numerous trials and studies conducted with TLR5 variants over the years, including advanced solid tumors, lung, colorectal, lymphoma, breast, uveal melanoma and bladder, along with radio-ablative sickness Studies have shown the potential to modify the microenvironment in liver, lungs, and bladder to suppress TLR5+ and TLR5- metastases, generate an adaptive immune response for prolonged antitumor effect, and protect from radio and chemotherapy side effects. We will look to initiate further TLR5 variant studies both as monotherapies and adjuvants.


